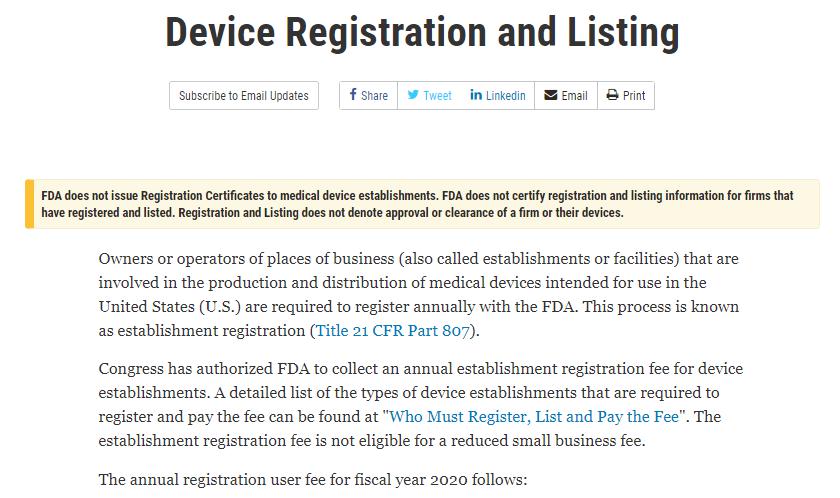
I-FDA ikhiphe isaziso esinesihloko esithi “ukubhaliswa kwedivayisi nokufakwa ohlwini” kuwebhusayithi yayo esemthethweni ngomhlaka-23 Juni, esigcizelele ukuthi:
I-FDA ayikhiphi Izitifiketi Zokubhalisa ezinkampanini zamadivayisi ezokwelapha. I-FDA ayiqinisekisi ukubhaliswa nokufakwa ohlwini
ulwazi lwezinkampani ezibhalisile futhi ezibhalisiwe. Ukubhaliswa kanye nokufakwa ohlwini akusho ukuvunyelwa noma ukuvunyelwa kwenkampani
noma amadivayisi abo.
Izinkinga okudingeka siziqaphele ekubhalisweni kwe-FDA yilezi ezilandelayo:
Umbuzo 1: iyiphi inhlangano ekhiphe isitifiketi se-FDA?
A: asikho isitifiketi sokubhaliswa kwe-FDA. Uma umkhiqizo ubhaliswe ne-FDA, kuzotholakala inombolo yokubhalisa. I-FDA izonika umfakisicelo incwadi yokuphendula (esayinwe yisikhulu esiphezulu se-FDA), kodwa asikho isitifiketi se-FDA.
Isimemezelo se-FDA sesaziso esinjalo ngalesi sikhathi siyisikhumbuzo esinamandla! Ngenxa yentuthuko yakamuva yesimo sobhubhane e-United States, isidingo semikhiqizo yokuvimbela ubhubhane lwezokwelapha ethunyelwa e-United States sikhule kakhulu, futhi isidingo sokubhaliswa kokuthunyelwa kwamanye amazwe naso sikhule.
Uma amanye amabhizinisi ezenza i-FDA ukuze akhiphe izitifiketi kubakhiqizi, amanye amabhizinisi okusabalalisa angathola “izitifiketi ze-FDA” mbumbulu lapho exhumana nabakhiqizi.
Umbuzo 2: Ingabe i-FDA idinga ilebhu eqinisekisiwe?
A: I-FDA iyi-ejensi yokuphoqelela umthetho, hhayi i-ejensi yesevisi. Uma othile ethi uyilabhorethri yesitifiketi se-FDA, okungenani udukisa abathengi, ngoba i-FDA ayinayo insizakalo yomphakathi.
Izikhungo zokuqinisekisa ucansi kanye nama-laboratory, azikho okuthiwa “i-laboratory ekhethiwe.” Njengenhlangano kahulumeni yokuphoqelela umthetho, i-FDA akufanele ihileleke ezintweni ezinjengokuba ngumpempe kanye nomdlali. I-FDA izohlola isevisi kuphela.
Ikhwalithi ye-GMP yelabhorethri izoqashelwa, futhi ofanelekayo uzonikezwa isitifiketi, kodwa ngeke "sibekwe" noma sinconywe emphakathini.
Umbuzo 3: ingabe ukubhaliswa kwe-FDA kudinga i-ejenti yase-US?
A: Yebo, ibhizinisi kumele liqoke isakhamuzi sase-US (inkampani/iNhlangano) njenge-ejenti yalo lapho libhalisa ne-FDA. I-ejenti inesibopho sezinsizakalo zenqubo ezise-United States, okuyizindaba ezixhumana ne-FDA kanye nomfakisicelo.
Amaphutha avamile ekubhalisweni kwe-FDA
1. Ukubhaliswa kwe-FDA kuhlukile esitifiketini se-CE. Imodi yayo yokuqinisekiswa ihlukile emodini yokuhlola umkhiqizo wesitifiketi se-CE + isitifiketi sokubika. Ukubhaliswa kwe-FDA empeleni kusebenzisa imodi yokumemezela ubuqotho, okungukuthi, unemodi yokumemezela ngokwethembeka imikhiqizo yakho.
Ngokuhambisana nezindinganiso ezifanele kanye nezidingo zokuphepha, futhi kubhaliswe kuwebhusayithi ye-US Federal, uma kwenzeka ingozi ngomkhiqizo, khona-ke kufanele uthwale umthwalo wemfanelo ohambisanayo. Ngakho-ke, ukubhaliswa kwe-FDA kwemikhiqizo eminingi, akukho ukuhlolwa kwesampula kokuthumela.
Nesitatimende sesitifiketi.
2. Isikhathi sokusebenza sokubhaliswa kwe-FDA: Ukubhaliswa kwe-FDA kusebenza unyaka owodwa. Uma kungaphezu konyaka owodwa, kudingeka kuthunyelwe kabusha ukuze kubhaliswe, futhi imali yonyaka ehilelekile nayo idinga ukukhokhwa futhi.
3. Ingabe i-FDA ibhalisiwe ngesitifiketi?
Eqinisweni, asikho isitifiketi sokubhaliswa kwe-FDA. Uma umkhiqizo ubhaliswe ne-FDA, kuzotholakala inombolo yokubhalisa. I-FDA izonika umfakisicelo incwadi yempendulo (esayinwe yisikhulu esiphezulu se-FDA), kodwa asikho isitifiketi se-FDA.
Isitifiketi esivame ukusibona sikhishwa yi-ejensi yomlamuleli (i-ejenti yokubhalisa) kumkhiqizi ukufakazela ukuthi isize umenzi ukuqedela "ukubhaliswa kwesikhungo sokukhiqiza kanye nokubhaliswa kohlobo lomkhiqizo" okudingeka yi-FDA.
(ukubhaliswa kokusungulwa kanye nokufakwa ohlwini kwamadivayisi), uphawu oluqediwe luzosiza umenzi ukuthola inombolo yokubhalisa ye-FDA.
Ngokusho kwamazinga ahlukene engozi, i-FDA ihlukanisa amadivayisi ezokwelapha ngezigaba ezintathu (I, II, III), kanti isigaba III sinezinga eliphezulu kakhulu lengozi.
I-FDA ichaze ngokucacile izidingo zokuhlukaniswa komkhiqizo kanye nokuphathwa kwedivayisi ngayinye yezokwelapha. Njengamanje, kunezinhlobo ezingaphezu kuka-1700 zekhathalogi yamadivayisi ezokwelapha. Uma noma iyiphi idivayisi yezokwelapha ifuna ukungena emakethe yase-US, kumele iqale icacise izidingo zokuhlukaniswa kanye nokuphathwa kwemikhiqizo efakelwe ukumaketha.
Ngemva kokucacisa ulwazi olungenhla, ibhizinisi lingaqala ukulungiselela izinto zokufaka isicelo ezifanele, bese libika ku-FDA ngokwezinqubo ezithile ukuze lithole imvume. Kunoma yimuphi umkhiqizo, amabhizinisi kudingeka abhalise futhi abhale imikhiqizo ohlwini.
Kwimikhiqizo yekilasi lokuqala (engaba ngu-47%), kusetshenziswa ukulawula okuvamile. Iningi lemikhiqizo lidinga ukubhaliswa, ukufakwa ohlwini kanye nokusetshenziswa kwezindinganiso ze-GMP, futhi imikhiqizo ingangena emakethe yase-US (imbalwa kakhulu yayo exhunywe ne-GMP)
Inani elincane kakhulu lemikhiqizo egciniwe kudingeka lifake isicelo esingu-510 (k) ku-FDA, okungukuthi i-PMN (isaziso sangaphambi kwemakethe));
Kumikhiqizo yesigaba II (engaba ngu-46%), kusetshenziswa ukulawula okukhethekile. Ngemva kokubhalisa nokufakwa ohlwini, amabhizinisi kudingeka asebenzise i-GMP futhi afake isicelo se-510 (k) (imikhiqizo embalwa ikhululiwe ku-510 (k);
Emikhiqizweni yesigaba sesi-3 (cishe u-7%), ilayisensi yokumaketha ngaphambi kokuthengisa iyasetshenziswa. Ngemva kokubhalisa nokufakwa ohlwini, amabhizinisi kumele asebenzise i-GMP futhi athumele isicelo se-PMA (isicelo sokumaketha ngaphambi kokuthengisa) ku-FDA (Ingxenye yesi-3)
I-PMN).
Ngemikhiqizo yesigaba I, ngemva kokuba ibhizinisi lithumele ulwazi olufanele ku-FDA, i-FDA yenza isimemezelo kuphela, futhi akukho sitifiketi esifanele esinikezwa ibhizinisi; kumadivayisi esigaba II nesesi-III, ibhizinisi kumele lithumele i-PMN noma i-PMA, futhi i-FDA izokwenza
Nikeza ibhizinisi incwadi esemthethweni yokuvuma ukufinyelela emakethe, okungukuthi, vumela ibhizinisi ukuthi lithengise imikhiqizo yalo ngqo emakethe yamadivayisi ezokwelapha ase-US egameni lalo.
Ukuthi kufanele uye ebhizinisini ukuze kuhlolwe i-GMP enqubweni yokufaka isicelo kunqunywa yi-FDA ngokuya ngezinga lobungozi bomkhiqizo, izidingo zokuphatha kanye nempendulo yemakethe kanye nezinye izici eziphelele.
Kusukela ngenhla, singabona ukuthi imikhiqizo eminingi ingathola isitifiketi se-FDA ngemuva kokubhaliswa, ukufakwa ohlwini lwemikhiqizo kanye nokusetshenziswa kwe-GMP yamadivayisi ezokwelapha, noma ukulethwa kwesicelo se-510 (k).
Ungahlola kanjani ukuthi umkhiqizo ubhaliswe yi-FDA noma ubhaliswe ku-510k?
Indlela ewukuphela kwayo enegunya: hlola iwebhusayithi ye-FDA
Isikhathi sokuthunyelwe: Jan-09-2021